Overview
An electrochemical cell is a device capable of either harnessing electrical energy generated from chemical reactions within it or using externally supplied electrical energy to drive chemical processes. These devices exhibit the unique ability to convert chemical energy into electrical energy and, conversely, to facilitate chemical reactions using electrical energy. The two main types of electrochemical cells are galvanic (voltaic) cells and electrolytic cells. Galvanic (Voltaic) cells convert chemical energy into electrical energy. They are commonly found in batteries. The electrons produced by the chemical reaction flow through an external circuit, creating an electric current. Electrolytic Cells are non-spontaneous electrochemical devices that require an external electrical energy source to drive a chemical reaction. Both types of electrochemical cells consist of two electrodes (conductors where the electrical current enters and exits the cell) and an electrolyte (a substance that facilitates the movement of ions between the electrodes). In the DEEP group, we are currently studying and developing electrochemical cells, including fuel cells, electrolyzers, and batteries.
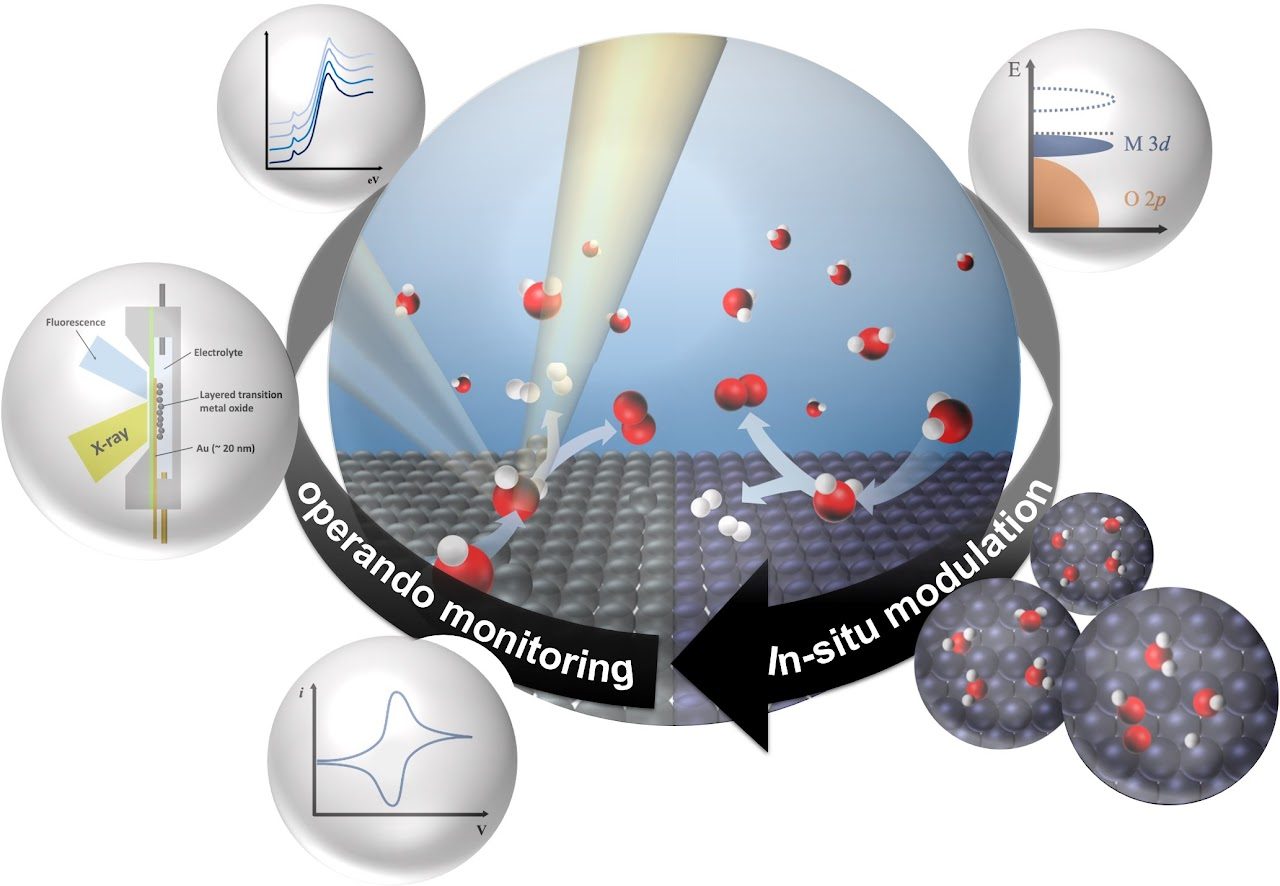
Water splitting for green hydrogen generation
The share of volatile renewable energy sources within our existing power system is on a steady incline, and this trajectory is expected to persist well into the coming decades. The coupling of water electrolysis with renewable electricity emerges as a highly promising method for producing green hydrogen. This approach not only amplifies the grid's flexibility but also extends the reach of renewable energy transmission across vast distances, thereby fortifying the large-scale expansion of renewable energy generation.
Water electrolysis encompasses two primary forms:
Aqueous Electrolysis: In this method, water is combined with an electrolyte, typically an acid or an alkaline substance
Solid Oxide Electrolysis: These cells function at elevated temperatures and employ a solid oxide electrolyte material, such as zirconia, rather than a liquid electrolyte.
The overarching reaction in water electrolysis can be summarized as 2H2O(l) → 2H2(g) + O2(g). Presently, our endeavors revolve around the development of high-performance and cost-effective water electrolyzers, poised to spearhead the generation of green hydrogen.
Chem 2023, 9, 1645-1657
Nature Catalysis, 2021, 4, 212-222
Nature Communications, 2021, 12, 5676
Chem. Soc. Rev., 2020, 49, 9154-9196
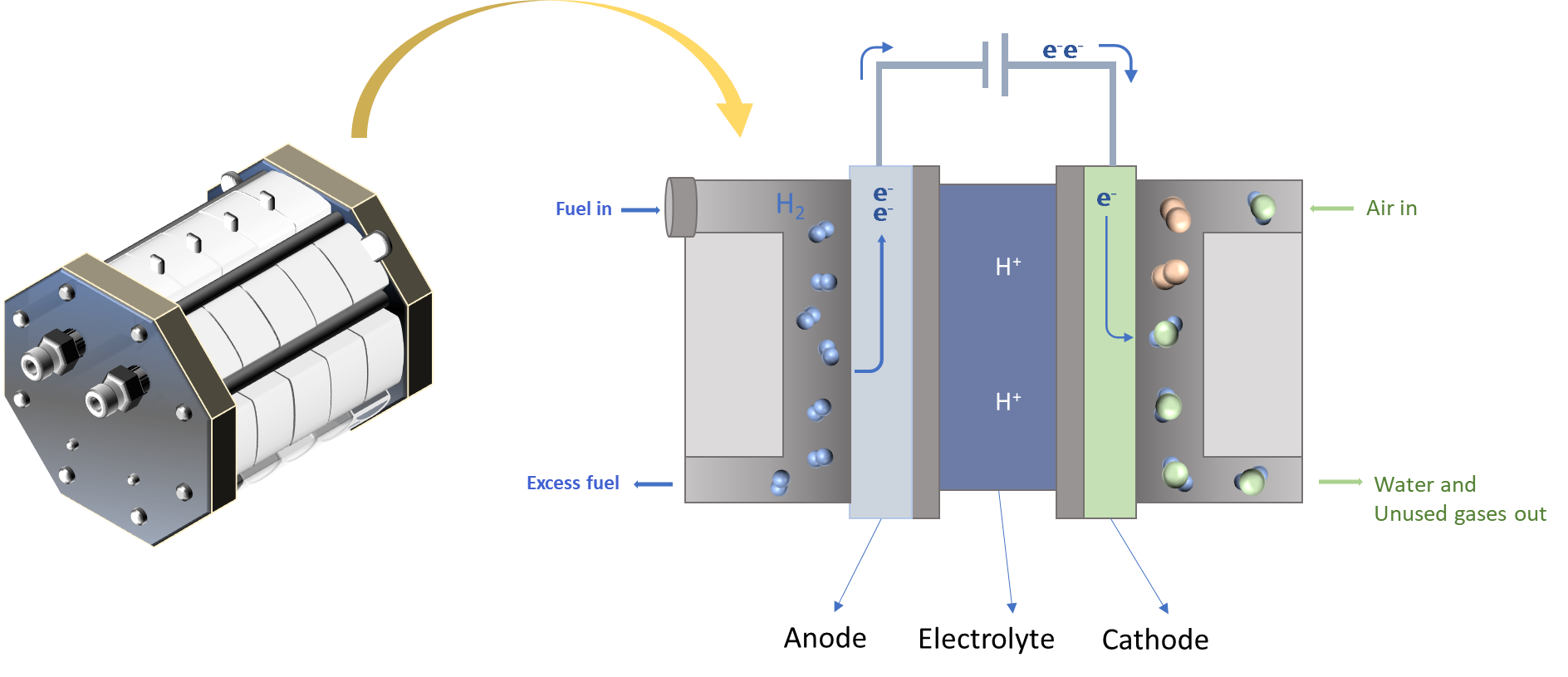
Fuel cells as a clean & efficient power source
Fuel cells are indeed remarkable electrochemical devices that play a pivotal role in generating electricity through the interaction of a fuel and an oxidizing agent, commonly oxygen or air. They are celebrated for their clean and efficient conversion of chemical energy into electrical power, with minimal emissions. This quality renders them highly valuable for a diverse array of applications spanning power generation, transportation, and portable electronics.
One of the key attributes that sets fuel cells apart is their impressive energy density. In comparison to batteries, which typically range from 0.1 to 0.27 kWh/kg, fuel cells, particularly hydrogen fuel cells, offer substantially higher energy density. For instance, hydrogen gas, a common fuel for these cells when stored at 700 bars pressure, boasts a remarkable energy density of 39.6 kWh/kg. This characteristic underscores the immense potential of fuel cells in applications where extended operation and high energy storage are paramount.
Fuel cells come in various types based on the electrolyte they employ, including alkaline fuel cells (AFC), proton exchange membrane (PEM) fuel cells, and solid oxide fuel cells (SOFCs), each catering to distinct applications and operating conditions.
At present, our focus is dedicated to the development of cost-effective electrodes engineered to deliver exceptional performance in fuel cell applications. This work holds the promise of advancing the efficiency and sustainability of fuel cell technology, contributing to a cleaner and more energy-efficient future.
Nature Energy, 2021, 6, 614-623
Nature Catalysis, 2022, 5, 777-787
Nature Energy, 2022, 7, 866-875
Nature Communications, 2021, 12, 5235
Batteries for challenging-condition applications
The rising demand for advanced energy storage solutions has set higher standards for batteries, particularly in terms of energy densities, fast charging capabilities, and thermal stability. To meet these evolving needs, we are actively engaged in the development of batteries designed to excel in challenging or demanding conditions.
Our current research endeavors are primarily concentrated in two areas: advanced metal ion batteries and metal-air batteries. These technologies hold the potential to address critical challenges in the energy storage field, from improving energy density to enhancing the charging process.
For instance, while electric vehicles (EVs) have gained widespread acceptance, the issue of prolonged charging times persists as a concern. Accelerating the charging rate is a logical solution, but it introduces complexities such as elevated overpotentials and accelerated degradation of electrode materials. To tackle this challenge, one of our ongoing projects is dedicated to elucidating the underlying factors that limit the extremely fast-charging capabilities of battery electrodes. Our aim is to pave the way for the development of fast-charging batteries that can redefine the EV experience and contribute to a more sustainable and efficient future.
ACS Energy Letters, 2023, 8, 7, 2986-2995
Advanced Materials, 2023, 35, 10, 2207076